7 Lab 7: Control of Microbial Growth
Lab Objectives
After completing this lab, the student should be able to:
- Use aseptic technique to transfer specimens for the UV test, temperature test, Kirby Bauer method, and disk diffusion method.
- Interpret growth patterns to determine antimicrobial effectiveness.
- Apply microbial cell wall structure to antimicrobial control.
- Examine the effectiveness of pigments and endospores to protect against the effects of UV radiation.
Introduction
Controlling microbial growth is key to medicine and to everyday life so there are many methods used to chemically and physically limit the growth of microbes. Control methods include inhibition (bacteriostatic) or elimination (bactericidal) microbes. While elimination is ideal, not all substances or surfaces can be treated sufficient to eliminate microbes and sometimes sterilization is not a practical method. Remember that control methods can reduce microbial populations or completely eliminate microbes thus leaving a sterile environment, food production, or surface. While sterility is more important in the clinic, decreasing the total microbial load is commonly used whenever surfaces are “cleaned” or foods are “prepared”.
The two main methods of controlling microbial growth are:
- Chemical methods which include the use of chemicals to eradicate or decrease the microbial load.
- Physical methods include filtration, temperature treatments, and radiation. These methods will either reduce the microbial load, completely remove microbes, or prevent microbes from reproducing thus resulting in cell death.
Chemicals are often convenient a convenient way to eliminate or reduce the number of microbes on inanimate surfaces (disinfectants) such as countertops, living surfaces (antiseptics) such as skin, or ingested (antibiotics). These chemotherapeutic agents come in a wide range of spectrums and thus have many uses. For examples, a compound that is a bactericide would kill bacteria but not fungi or viruses while a fungicide would kill fungi but not bacteria or viruses. Sporicides are compounds that typically kill endospores. A compound may also be broad spectrum where it can target multiple groups of microbes (or microbes and endospores) or narrow spectrum (only targeting one class or group of microbes). For examples, natural penicillins are effective against Gram positive bacteria but offer little effectivity against Gram negative bacteria and certainly no protection against fungi, viruses, endospores, or other groups of microbes.
Physical control methods are quite a bit less discriminatory in their targeted groups and are thus considered broad spectrum. Most methods of physical control target DNA, proteins, or size which are shared in common across the microbial spectrum.
- Radiation causes mutations, specifically the formation of pyrimidine or thymine dimers, which prevent DNA replication – one of the key checkpoints in the cell cycle.
- Heat breaks the hydrogen bonds that keep proteins and nucleic acids in their functional shapes. As such, many organisms cannot carry out life processes upon exposure to heat. Heat comes in two forms – moist heat which provides are more efficient transfer of heat from the environment to the organism(s) and dry heat which does not rely upon moisture. As such, dry heat methods such as ovens typically require longer times and/or higher temperatures to kills microbes and are basically useless against endospores. Examples of moist heat include autoclaving, boiling, and pasteurization processes. [Note: Ultra high temperature (UHT) pasteurization is used to sterilize certain foods such as milk.]
- Low temperatures such as refrigeration and freezing slow or prevent microbial growth. While not true for endospores, freezing can damage the plasma membranes when ice crystals form.
- Filtration is a common method of physical control used for liquids that cannot be heat treated or air samples. During filtration, the air or liquid is passed through a filter which physically prevents particles (including microbes) from passing based on size. Not all filters are the same size so there is a limit as to the group of microbes that are targeted. For example, most bacteria would be removed from a solution if a 0.22um filter were used, however, this size would not remove viruses.
Microbes have developed some methods for counteracting physical and chemical methods of control. Some microbes are naturally resistant against chemicals and heat while others have had to develop mutations that degrade chemicals thus making the chemicals inactive. Likewise, endospore formers, bacteria that produce a pigment (color, specifically observed during the growth of colonies), or microbes that have DNA repair systems are more resistant to radiation treatment. Pigments act as a form of “sunscreen” by blocking some radiation while repair systems such as the SOS system fix any damage that radiation causes. Repair systems are limited in the amount of damage that they can fix – too many thymine dimers will result in cell death. Endospores are resistant structures due to their thick spore coats and their lack of metabolism, thus they are resistant to radiation. Heat shock proteins are a special group of proteins that help repair/ refold damaged proteins after a stress event, including temperature extremes.
This lab explores the use of physical and chemical methods of control on microbes. Specifically, we will look at exposure to different temperatures, UV radiation, antiseptics, disinfectants, and antibiotics.
Method (Lab@Home)
For this lab you will need:
7 NA plates
Cotton swabs
Bathroom cup
Clean water
Sharpie marker
Antiseptics
Disinfectants
Antibiotic data
Antibiotic Sensitivity table
Timer/ watch
Aluminum foil
Ziplock bag
Ruler with mm
Before beginning this lab exercise, you should thoroughly wash your hands with warm soap and water and wipe down your bench using the provided disinfectant.
1. Inoculate the 4 NA plates for UV radiation test.
- Using the bathroom cup, collect a small amount of clean water. (Bottled or purified water works better than tap water.)
- Dip a clean cotton swab in the water to moisten the swab, then collect a small amount of growth from your bacterial plate from the first lab.
- Spread bacteria on the entire surface of the NA plate by streaking top to bottom, left to right, and then at a 45⁰ angle across the surface of the plate. The goal is to cover every surface of the medium with the broth so that you can easily see where the treatment method damages bacterial cells and prevents growth.
- You will repeat this inoculation method for all four plates.
- Open all plates to sunshine (not at a window but in direct sunshine) or under a UV light for 2.5min or 5 min as demonstrate below. Two plates will be treated for 2.5min and two plates for 5 min.
- Cover the plates and put one plate for each time (2.5min and 5 min) in a dark cabinet or cover in foil while the cells grow. The other pair of plates will be kept in the light. [You are looking at the effect of UV exposure time and the effect of light depended repair systems.]
- Growth the plates at room temperature for 4 days.
2. Inoculate the 2 NA plates for temperature test.
- Inoculate 2 NA plates for confluent growth as described in the above procedure (Procedure 1).
- Place one plate in a zip lock bag and half seal. Incubate the plate at 4⁰C refrigerator for 4 days.
- Place the second plate at room temperature and grow for 4 days.
3. Inoculate a NA plate for Disinfectant and Antiseptic testing.
- Swab 1 NA plates with your organism using the streak for confluent growth method as described above (in Procedure 1).
- Select 2 antiseptics and 2 disinfectants that you have at home.
- Examples of antiseptics could be hydrogen peroxide, isopropyl alcohol, hand soap, body soap, shampoo, hand sanitizer.
- Examples of disinfectants would include surface cleaner, disinfectant wipe (or the liquid from a disinfectant wipe), Lysol, bathroom cleaner, glass cleaner.
- Record the names of the test compounds below.
Antiseptic 1 (A1): ____________________ Antiseptic 2 (A2): ____________________
Disinfectant 1 (D1): __________________ Disinfectant 2 (D2): ____________________
- It may be helpful to pour/ spray a small amount of each antiseptic and disinfectant into a separate clean bathroom cup.
- Using a clean swab, dip the swab into the antiseptic or disinfectant compound and dot onto the inoculated plate according to the diagram below. You should have one dot for each test compound.
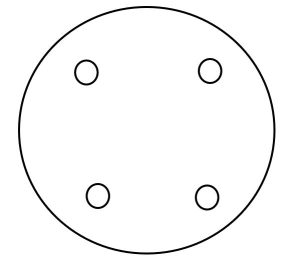
- Once your plate has been inoculated, incubate your plate at room temperature for 4 days then measure the zones of inhibition created by your test compounds.
4. Analyze a Kirby Bauer dataset.
- Download the data set from the Kirby Bauer test and the Antibiotic Sensitivity table from BBL. [Both documents are available on Canvas.]
- Record the zone of inhibition measurements to your table in the Results section.
- Using the Antibiotic Sensitivity table from BBL, decide if each organism is S (sensitive), I (shows intermediate sensitivity), or R (resistant) for each antibiotic tested. Record your data in the provided Table in the Results section.
5. Clean up
After you have finished your inoculations, wipe down your work area with disinfectant and wash your hands. Blow out the candle and store for later.
Once you have read your plates (after their 4 day incubation), wrap plates in a plastic bag and dispose of in the trash. Again, wipe down your work area and wash your hands.
Results
Because microbes require time to grow, you will view your results after 4 days.
Develop a plus system to convey the amount of growth per treatment condition. How many + marks would you give the following descriptions?
No growth = ______ Little growth = ________
Moderate growth = ______ Heavy growth = _______
Table 1. Results from UV Exposure.
|
Plate
|
Growth @ 2.5min
|
Growth @ 5min
|
|
Bacterium
|
|
|
Rank the growth in each plate based on your plus system.
Table 2. Results from Growth at Different Temperatures.
|
Organism
|
4⁰C
|
21⁰C
|
|
Bacterium
|
|
|
|
S. marcescens
|
|
|
Rank the growth in each plate based on your plus system.
Many times, chemical agents will produce zones of inhibition where an organism will not grow around a compound. For each of your plates (Disk Diffusion and Kirby Bauer tests), measure the zones of inhibition (ZOI) and report in the tables below.
Table 3. ZOI Measurements Produced by the Disk Diffusion Test. All measurements are reported in mm.
|
Test Compound
|
Bacterium
|
|
Antiseptic 1
|
|
|
Antiseptic 2
|
|
|
Disinfectant 1
|
|
|
Disinfectant 2
|
|
Table 4. ZOI Measurements Produced by the Kirby Bauer Test. Record the zones of inhibition measurements for each antibiotic. (R = Resistant, I = Intermediate, S = Sensitive, [Abx] = Antibiotic Concentration). This data requires the use of the provided Antibiotic dataset and the Antibiotic Sensitivity table.
|
Organism
|
Disk Code
|
[Abx]
|
Zone of Inhibition (mm)
|
Interpretation (R, I, S)
|
|
E. coli
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P. aeruginosa
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S. aureus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

