2 Lab 2: Isolation Streak and Types of Media
Lab Objectives
After completing this lab, the student should be able to:
- Use aseptic technique to transfer microbes to different media types without contamination.
- Apply the isolation streak to separate a mixed culture into pure colonies.
- Describe when general purpose, chemically defined, enrichment, selective, and differential media should be used.
- Describe how the isolation streak method is an example of sample dilution.
Introduction
The isolation streak, like aseptic technique, is a key method in microbiology that is used to separate mixed cultures through a dilution process so that pure cultures can be obtained. In this lab, you will learn how to perform a four-quadrant isolation streak though it should be noted that two-, three-, and even five-quadrant streaks may be used within the clinical microbiology lab setting. The number of quadrants streaked is dependent upon the suspected number of bacteria in a sample – if a sample it though to have low numbers of bacteria (e.g. CSF sample) then a two-quadrant streak may be used verses a sample that has a high bacterial cell density (e.g. fecal sample) which may use a five-quadrant streak.
It should also be noted that bacterial growth can be further influenced by the type of culture media. Not only does culture media come in different forms (broths, deeps, plates, or slants) as discussed in Lab: Aseptic Technique, but it also comes in different types or classifications. While media cannot exactly mimic the
- Chemically defined (synthetic) media – a media where the exactly chemical composition is known and ingredients are typically salts (not just NaCl). Chemically defined media are useful for determining the nutritional growth requirements of microbes.
- General purpose (complex or rich) media – a media where the exactly chemical composition is not known due to differences in some ingredients that can vary by preparation. General purpose media is often used to grow a wide variety of microbes and would include such media as nutrient broth/ agars or tryptic soy broths/ agars. A key give away that a medium is a general purpose medium would be the use of digests, tryptone, or extracts to make the media.
General purpose media and chemically defined media can be difficult to separate. The key is in that general purpose media will contain extracts, digests, or tryptone while chemically defined media are very detailed ingredients typically in the from of salts. Here’s a comparison to help illustrate this difference.
Table 1. A comparison of a chemically defined Simmons Citrate Agar and general purpose Nutrient Agar.
|
Medium
|
Simmons Citrate Agar1
|
Nutrient Agar2
|
|
Type
|
Chemically defined
|
General purpose
|
|
Ingredients |
Ammonium dihydrogen phosphate Dipotassium phosphate Sodium chloride Sodium citrate Magnesium sulfate Bromthymol blue Agar |
Pancreatic digest of gelatin Beef extract Agar |
|
What makes it the type |
All ingredients with the exception are agar – that is cation + anion. |
Digest and extract will vary by lot due to processing therefore the exact nutrients will not be the same each time the medium is made. |
Sources: 1BD. (2015). BBL Simmons Citrate Agar Slants. 2BD. (2014). BBL Nutrient Agar product Information.
General purpose media can be further divided into more specific classifications based on the function of the media.
- Differential media allows for the classification or grouping of microbes based on their ability to utilize nutrients in the medium and/ or change colony appearance of the microorganism or of the medium. Common examples of Differential media that you may encounter are blood agar, eosin methylene blue (EMB) agar, MacConkey (MAC) agar, or mannitol salt agar (MSA).
- Enrichment media encourages the growth of some species while omitting selective pressure on other species. In this media type, blood or serum products are frequently used to support the growth of fastidious organisms – those that grow only when specific nutrients are present. A good example of an enrichment medium would be blood agar or chocolate agar (blood agar that uses lysed blood).
- Selective media actively selects against some species by containing ingredients that limit or prohibit the growth of some microbes. Examples of selective media would be MSA, EMB, or MAC. For selective media, good growth will produce easy to see colonies while stunted growth will produce pinpoint sized colonies that show the organisms is having difficulty growing under selective pressure of the medium. Some organisms may not be able to grow at all under the selective pressure, thus producing no growth or no colonies.
You may have noticed that some agars such as blood agar, MSA, EMB, and MAC can serve more that one role. Let’s take a look at how these media meet the criteria of being more than one type of general purpose media.
Table 2. Breakdown of differential, enrichment, and selective properties of selected medias.
|
Medium
|
Differential
|
Enrichment
|
Selective
|
|
Blood agar
|
Blood hemolysis pattern |
Serum in blood |
——— |
|
Chocolate agar
|
——— |
Serum in lysed blood |
——— |
|
EMB
|
Lactose fermentation |
——— |
Bile salts + eosin dye |
|
MAC
|
Lactose fermentation |
——— |
Bile salts + crystal violet |
|
MSA
|
Mannitol fermentation |
——— |
NaCl concentration |
In today’s lab, you will be using the four-quadrant isolation streak.
Method (Lab@Home)
For this lab you will need:
Petri plate
Cotton swabs
Sharpie marker
Candle
Lighter or matches
Paper towel
Instructions for Isolation Streak of Mixed culture onto Nutrient Agar.
Before beginning this lab exercise, you should thoroughly wash your hands with warm soap and water, wipe down your work area with a disinfectant, and light a candle.
Because you will be working around a flame, be sure to tie back any long hair or clothing.
1. Label a NA plate with your culture information (“mixed culture”), date, and initials. You may also find it helpful to label four quadrants on your plate I-IV while you are learning to streak for isolations.
- Using a clean cotton swab, collect a small amount of growth (colonies) from your bacterial plate from the last lab.
- Open the lid (the side without the growth medium) of the plate and carefully hold over the Petri plate while you streak the agar surface in the first quadrant. You should streak three or four times across the first quadrant. **Agar can be somewhat soft – think of jello – try not to rip or tear the agar with your cotton swab.** Replace the plate lid after inoculating.
- Set the used cotton swab on a piece of paper toweling to be disposed of at the end of the lab.
- Beginning in first quadrant, use a second cotton swab to pull cells into the second quadrant. You will streak two times across the second quadrant pulling cells from the first quadrant.
- Place the used cotton swab on the paper toweling.
- Beginning in second quadrant, use a third cotton swab to pull cells into the third quadrant. Again, you will streak two times across the third quadrant pulling cells from the second quadrant.
- Set aside the used (third) cotton swab for later disposal.
- Beginning in third quadrant, use a fourth clean cotton swab to pull cells into the fourth quadrant. Again, you will streak three or four times across the fourth quadrant pulling cells from the third quadrant. Be careful not to overlap the first and fourth quadrant as this often prevents isolated colonies from forming.
- Place the plate bottoms up (inverted) in a warm area where it can grow for 2-3 days.
- Wrap the used cotton swabs in the paper toweling. Wipe down your work area with disinfectant. The paper toweling containing the cotton swabs can then be wrapped in the disinfectant wipe (or sprayed with disinfectant) before disposing of in the trash.
- Wash your hand and blow out the candle.
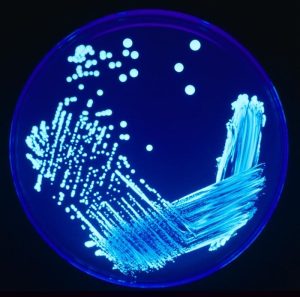
Figure 1. Four Quadrant Isolation Streak Method. Image source: James Gathany, CDC 2005. Image number 7925.
Results
Because microbes require time to grow, you will view your results in 2-3 days.
In the space below, draw a picture of your isolation streak (after it has grown). You may want to use colored pencils/ pens/ highlighters to distinguish between the different colonies.
Did you achieve isolation (of colonies)? Yes No
These data tables are included as a summary of the different media mentioned in this lab’s background material. You will want to be familiar with these reactions to help answer questions on your lab report and lab quiz.
Table 1. Interpretation of the results from Selective and Differential Media Inoculations.
|
Plate
|
Selective Interpretation
|
Differential Interpretation
|
|
EMB
|
Gram negatives = growth
Gram positives = no growth or stunted colonies |
Metallic green, purple, black, or pink colonies = Lac fermented
Colorless colonies = no Lac fermented |
|
MAC
|
Gram negatives = growth
Gram positives = no growth or stunted colonies |
Pink colonies = Lac fermented
Colorless or tan colonies = no Lac fermented |
|
MSA
|
Halophiles = growth
Nonhalophiles = no growth or stunted colonies |
Yellow ring around agar = Man fermented
No change in media = no Man fermented |
Table 2. Interpretation of the results from Enrichment and Differential Media Inoculations.
|
Medium
|
Enrichment
|
Differential
|
|
SBA
|
Growth (no selective pressure) |
Hemolysis pattern
|

